High-quality water is essential for a variety of industrial finishing processes, but is too often overlooked. Discover where reverse osmosis fits into your finishing operation, including what it is, how it works and how to get the most out it.
Industrial-size reverse osmosis units like the ones pictured here are used to generate pure water for a variety of finishing processes.
For several surface finishing processes, a high-quality water supply is key to success. Reverse osmosis (RO) is one water treatment technique that has proven itself as a means of not only maintaining water quality but also as a way to reclaim valuable process chemicals and reduce fresh water usage.
Especially with industrial companies that go through large volumes of water, RO systems provide extreme cost savings for water and sewer costs as well as savings on additional labor and manpower required to meet pollution compliance standards.
Manufacturer and industry leader of wastewater and water treatment systems, Therma-Tron-X (TTX) provides this brief guide for those hoping to better understand or optimize their units.
Reverse Osmosis Use Cases
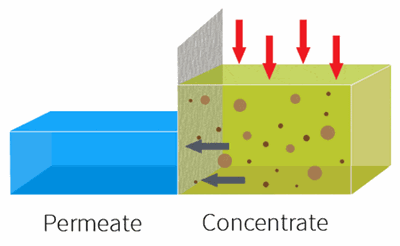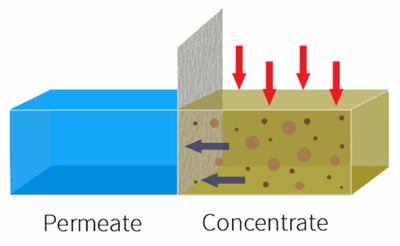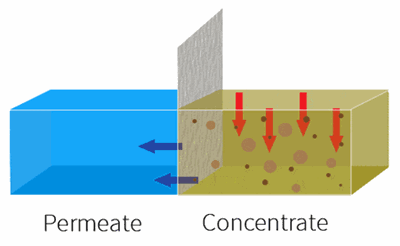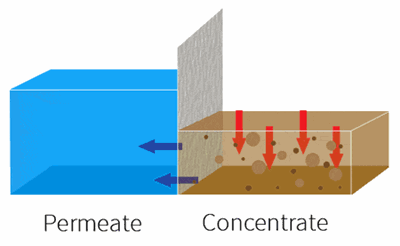
By applying pressure, the feed water is forced through the semi-permeable membrane.
RO is used to remove dissolved solids and contaminates from water used in both commercial and industrial applications by applying pressure to move water through a semi-permeable membrane.
In comparison to other methods of filtration such as nanofiltration, ultrafiltration or microfiltration, reverse osmosis can effectively remove contaminants as small as 0.0001 microns, including salts, ions and other materials.
Systems can be configured to treat and recycle wastewater generated by:
- Metal finishing and plating operations
- Circuit board and semiconductor manufacturing
- Automotive manufacturing
- Food and beverage production
- Groundwater and landfill leachate processes
RO systems can be used to remove low-molecular-weight resins and dissolved salts from things like electrocoat paint ultrafiltrate and can play a major role in reducing the consumption of DI water and generating less wastewater. It can be used to create a closed-loop system for a variety of surface finishing applications.
In electrocoat applications, it is not uncommon for a process to use a 2nd stage pass of RO in order to polish the product within tight tolerances. Afterwards, the water can be re-used in any part of the cycle. The systems can also help electrocoaters with paint recovery, reducing amount of wasted paint.
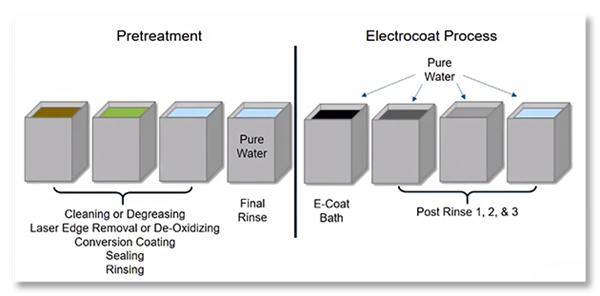
In this example of a pretreatment and ecoat line, pure water is required for several steps in the process.
For electroplating processes, RO systems have also played a role in the recovery of metals from plating rinse waters, which requires extremely high-quality water. Not only do platers face harsh requirements for metal discharge limits, but they must pay close attention to wastewater regulations.
High-quality water is especially important from a corrosion-performance perspective for pretreatments, as it is the last to touch the part before it’s coated and sealed. Having that water free of contaminants is very important for part quality and corrosion prevention. For example, powder coating usually requires a pretreatment to clean the part, which is then dried off before applying the powder. Poor water quality can result in water spots left from dissolved solids on the part after it leaves pretreatment, and this can lead to corrosion. RO can effectively eliminate that.
Generally speaking, RO systems can accomplish three different functions:
- Remove purified water from a feedwater stream (generating permeate)
- Reduce volume of wastewater in specific applications (minimizing concentrate)
- Selectively separate small ions and molecules from process stream
As a more cost-efficient alternative to deionization (DI) and ion-exchange systems, RO membranes can remove more bacteria and suspended solids without the use of costly chemicals. While DI systems can produce pure water as low as 1 microseimens, most water quality requirements can be easily met by RO units.
Water Quality
Surface finishers may know that they need high-quality water for their processes. But what they might not know is what level of quality is needed and how to measure it.
Identifying contaminants in your water is the first step to treating it for your company’s specific applications. Water tests are used to measure factors such as SDI, TDS, pH, ORP conductivity and other characteristics. Your source of water can have a major impact on the performance of the membrane filter, ultimately affecting the RO system as a whole. This can vary from water source to source, from city to city.
Water contamination can take various forms. Salts in potable water, such as iron, calcium, magnesium, chlorides, sulfates, phosphates, silicates, etc. can destroy plating baths if allowed to concentrate over time.
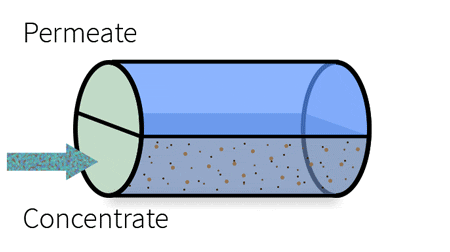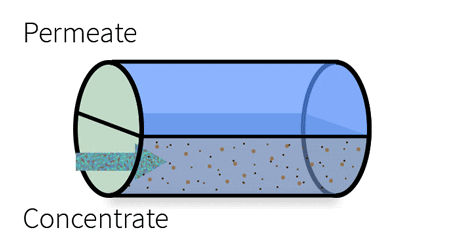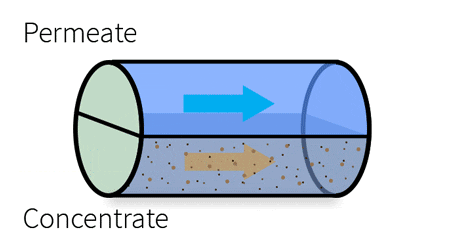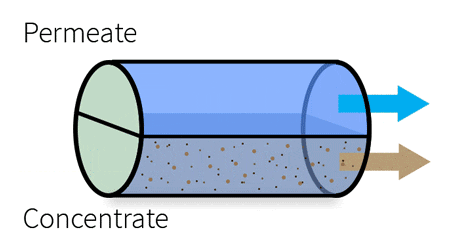
With industrial RO systems, the feed is essentially “split” into the permeate (clean water) and the concentrate (waste water).
The rate of water recovery can be calculated by taking into consideration water costs, ideal water usage and is then balanced to projected membrane life. For instance, a system could strive to achieve 90% permeate recovery but at the cost of membrane life, which can get costly.
Not every finishing processes holds the same requirements for water purity, and not every RO system is the same. Based on your application and water purity needs, sometimes a pilot test is needed to ensure the RO system meets the needs of the operation and is implemented correctly.
Rule of Thumb: Permeate and concentrate ratios can be altered based on customer needs, but usually start in the range of roughly 70% water recovery and 30% concentrate.
First, TTX consults the customer on the type of application, whether or not there are any unique components or rigid requirements—such as with semiconductor applications that require ultra-pure water—followed by looking at footprint and space available. Then, TTX conducts some water testing with the incoming water source and uses software to make projections, checking the projected system against all of the collected data. Based on the results, they can then determine exactly how many and what kind of membranes the system will require.
Membrane Maintenance
You might think of a membrane simply as a filter, but it’s much more than that.
One of the common challenges in maintaining a RO system is cleaning or replacing membranes. RO system cleaning typically occurs between 1-4 times a year. Improper maintenance of the membranes eventually leads to membrane fouling, which can lead to higher operating pressures, higher operating costs and poor water quality. This includes removing bio-slime with a high-pH cleaning and using a low-pH cleaning to remove scale.
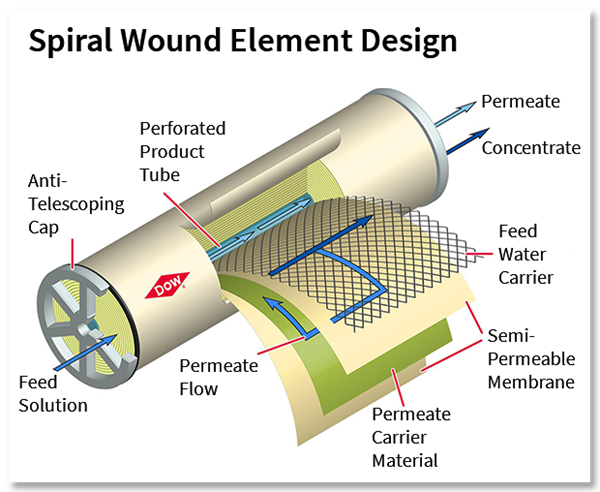
This cross-section demonstrates the components of a spiral wound membrane configuration, most popular for its compact design.
In addition to lowering system efficiency, excessive replacement of membranes can get costly. However, effective system installation, pretreatment and good maintenance habits can ultimately prevent system failure and minimize future maintenance requirements.
Advances in technology in the last three years have made a tremendous impact on the performance of membranes, and from a cost perspective, can lead to huge savings when compared to older or outdated membranes.
There are several different types of membrane designs available but typically, spiral wound is the most popular design because it’s the most cost effective and space efficient, offering a lot of membrane area in a small package.
The membrane surface itself consists of an ultra-thin barrier layer, a microporous substrate and a reinforcing polyester fabric, which make up the thin film composite membrane. This semi-permeable membrane is housed in a cylindrical, spiral-wound system, which carries the water feed as it moves through several layers before discharging the permeate and the concentrate.
Membranes can vary in pore size and thickness, giving a different level of salt passage and flow. It’s important to match the membrane to the design of the system because you’ll get huge differences in the performance of the membrane and ultimately the performance of the water you get out of the system.
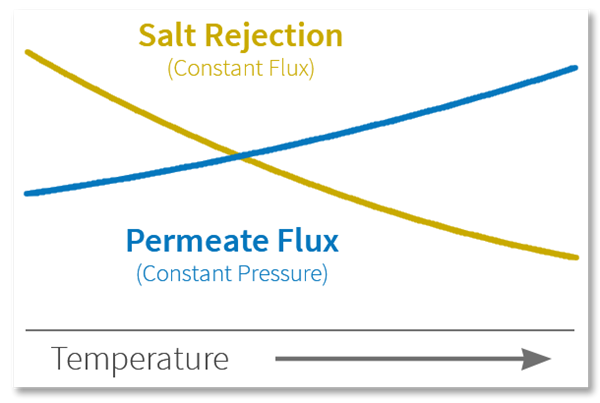
Rule of Thumb: For every 1°C increase, the permeate flow will increase approx. 3% and the salt passage increases approx. 6%.
There are several feedwater characteristics that can affect the performance of the membranes. This includes source concentration (i.e. feed water with high limestone concentration), temperature, osmotic pressure and pH.
Temperature is particularly important for membrane performance because RO systems are often designed around your minimum water temperature as well as the maximum. The reason that range is important is because on one hand cold water requires higher driving force than warm water, so the feed pump is sized for the cold water situation. On the other, warm water allows much higher salt water passage through the membranes. So the higher the temperature, the higher the permeate flow and the higher the salt passage.
Pretreatment of the water prior to use is also a major component to membrane health. Historically, systems have used water softeners and carbon filters, but newer technology (consistent with the last 15 years) uses chemical antiscalant injections to achieve the same results.
Using chemical injections as opposed to water softeners, the total cost of ownership and maintenance is dramatically reduced. Particularly with a system requiring a heavy flow, the costs of water softeners can add up, so chemical injection has quickly become a better alternative. TTX RO systems, including those used for commercial applications, all include a chemical feed directly built in.
RO System Components and Design
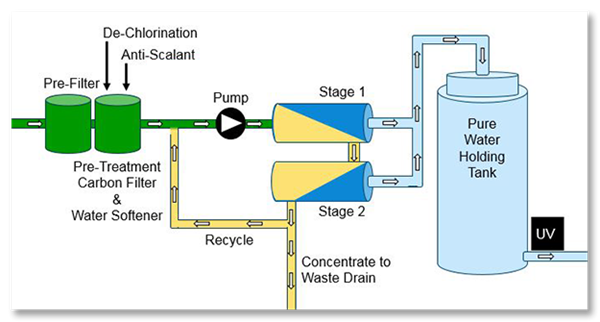
Common RO units include a strainer, pressure booster pump, cartridge filter and RO membrane modules. The strainer is designed to remove suspended solids from the feed solution, protecting the pump. The booster pump increases the feed solution pressure and cartridge filters remove particulates that can clog the units.
The most simplified system comes with its own pump, it will pressurize the water coming in to your membrane array and the membrane with split the stream to get permeate out (which may go to a storage tank for use later) and the concentrate, which is then disposed.
This is an example of a common reverse osmosis system and some of the system components it requires.
Looking at all aspects of design and requirements, RO systems are known for being cost-efficient. Units are compact, lowering installation costs. Power is only needed for the pump, lowering energy costs. Operation and labor requirements are minimal, further reducing costs.
Industrial units are designed prevent scaling when used as a medium in heat exchange functions. TTX Environmental RO systems automatically compensate for temperature and also integrate a chemical feed system for antiscalant and chlorine reduction.
With TTX, customers can specify exactly the components they need, including everything from membranes to PLCs and HMIs that fit the customer’s specs.
Here are a few questions to ask for your RO system design:
- What is the source of the feed water (well, municipal etc.)
- What is the feedwater temperature, does this fluctuate? (min. and max.)
- What is the feedwater pressure? (min. and max.)
- What is the daily volume of water required per day? Per hour?
- What are the water quality or purity requirements? Does it need to meet specific standards such as US EPA, ASTM, etc.?
- Where will the reject water concentrate from the RO system go?
- How much space is available for the system?
Systems that Last
Pictured here is a TTX installed industrial reverse osmosis unit with control panel.
Therma-Tron-X Environmental, a division of Therma-Tron-X, manufactures reverse osmosis units for virtually any application, commercial or industrial. These units can be used to provide essential clean water for manufacturing procedures with even the strictest requirements, all with minimal operator oversight.
TTX Environmental reverse osmosis units generate pure water for a variety of processes by separating dissolved solids from water with no carbon filters, no water softeners and with a lifetime supply of membranes. The systems use the latest in membrane and ion exchange technology. For pollution control standards, TTX Environmental helps plants meet compliance with federal, state and local discharge standards by using wastewater technology and process bath reclamation.
Original Source: https://www.pfonline.com/articles/how-to-optimize-your-reverse-osmosis-system-for-surface-finishing
Original Date: 10/22/18









/cdn.vox-cdn.com/uploads/chorus_image/image/61882977/GettyImages_872586288.0.jpg)